IN THE RANDOMIZED, PLACEBO-CONTROLLED PIVOTAL TRIAL
Joenja safety profile1,2

 Not actual patients.
Not actual patients.
Adverse reactions reported by ≥2 Joenja-treated patients and more frequently than placebo
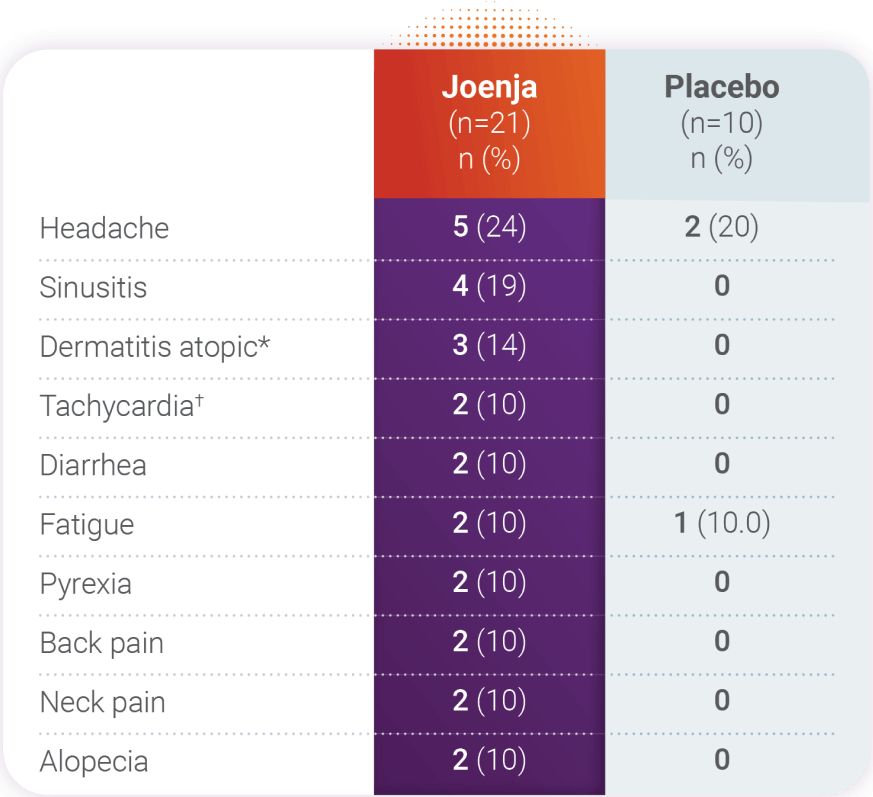
- No serious adverse drug reactions
were reported - No patients withdrew due to an adverse
drug reaction - The most common adverse reactions (>10%) were headache, sinusitis, and dermatitis atopic
*Dermatitis atopic: including dermatitis atopic and eczema.
†Tachycardia: including tachycardia and sinus tachycardia.
ANC, absolute neutrophil count; OLE, open-label extension.
About APDS
Hyperactivity along the Pl3Kδ signaling
pathway disrupts immune cell balance,
causing immune deficiency and
immune dysregulation.1,2,4,7
The extension
study
of Joenja is under investigation in
the open-label extension study with
some patients receiving treatment
for as long as 5 years.1,6
Registry. Front Immunol. 2018;9:543. doi:10.3389/fimmu.2018.00543 5. Rao VK, Webster S, Dalm VASH, et al. Effective “activated PI3Kδ syndrome”—targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017;130(21):2307-2316. doi:10.1182/blood-2017-08-801191 6. US National Library of Medicine. ClinicalTrials.gov/NCT02859727. 7. Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866-871. doi:10.1126/science.1243292