Joenja is the first and only selective PI3Kδ inhibitor indicated for the treatment of APDS1-4
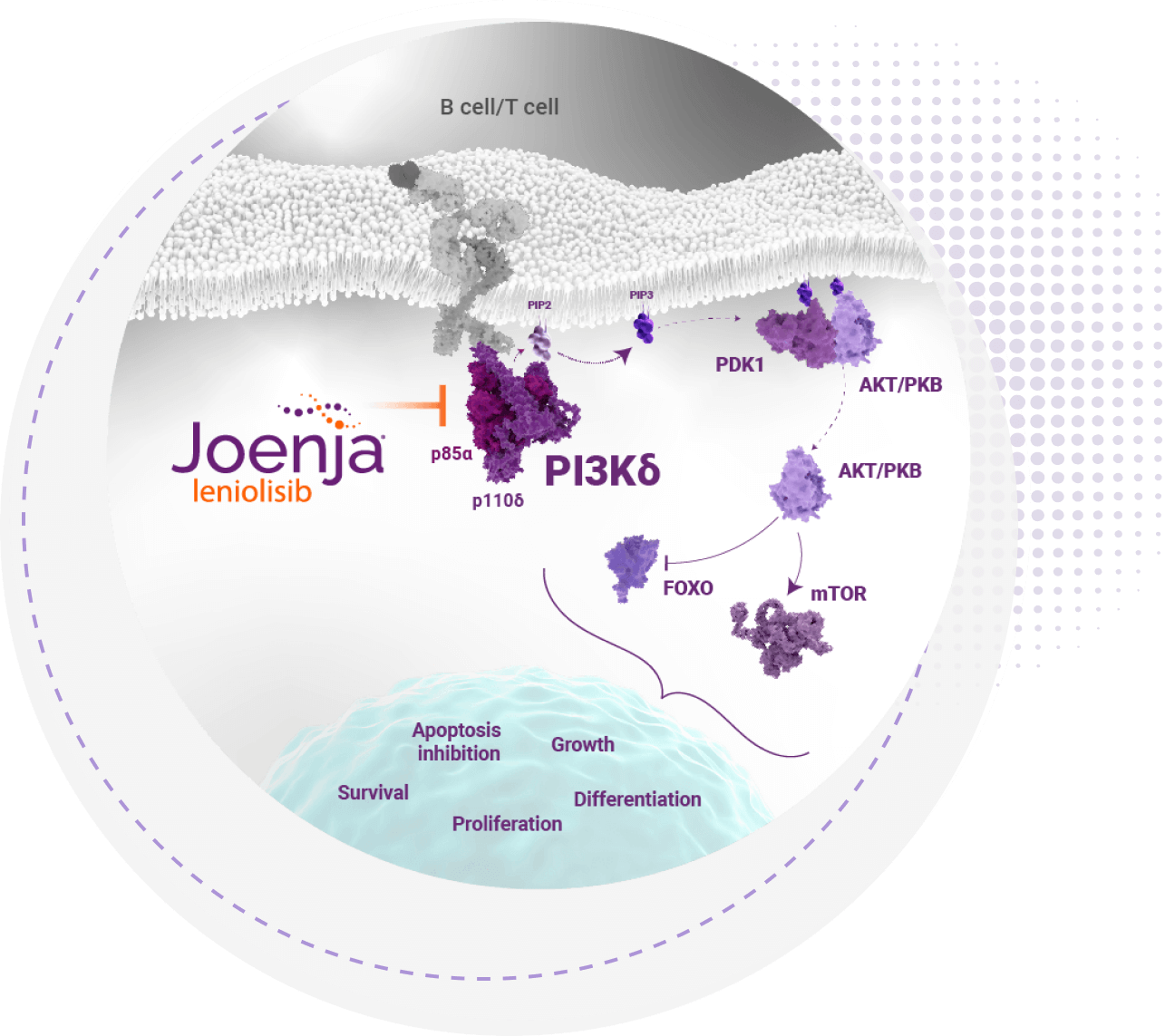
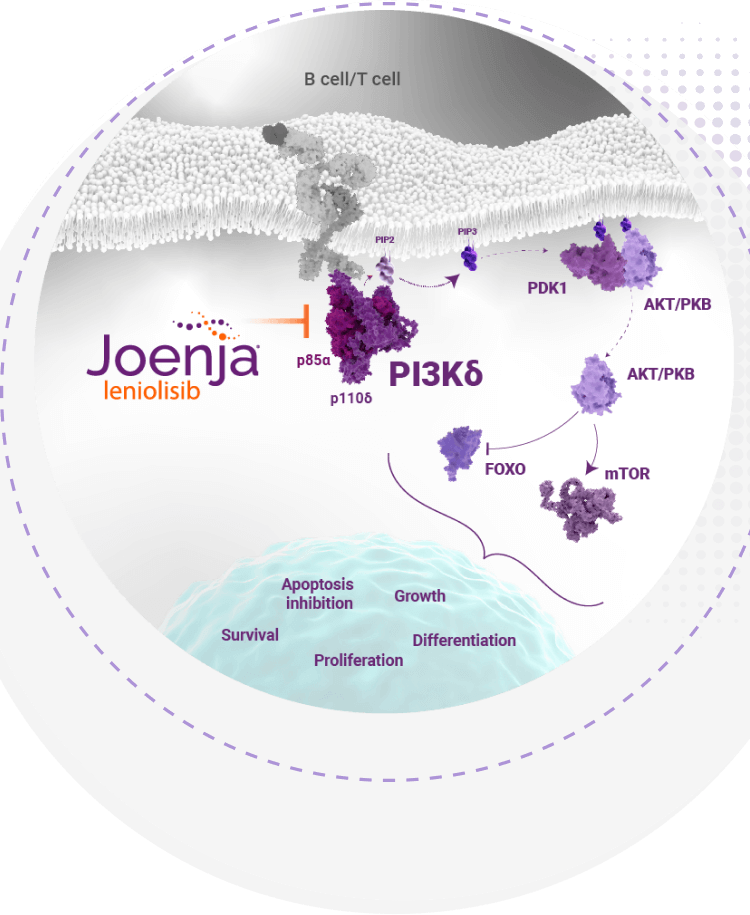

defect caused by APDS to help normalize the
hyperactive PI3Kδ pathway4-6
Joenja facilitates a balanced PI3Kδ pathway to support proper immune function1,7
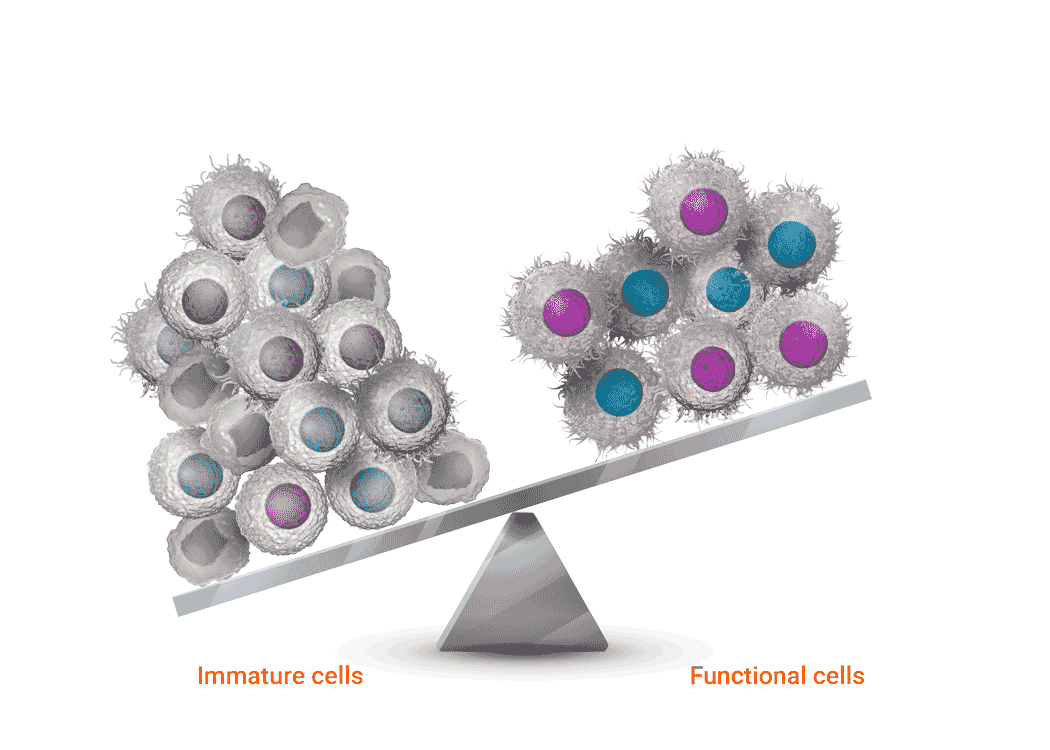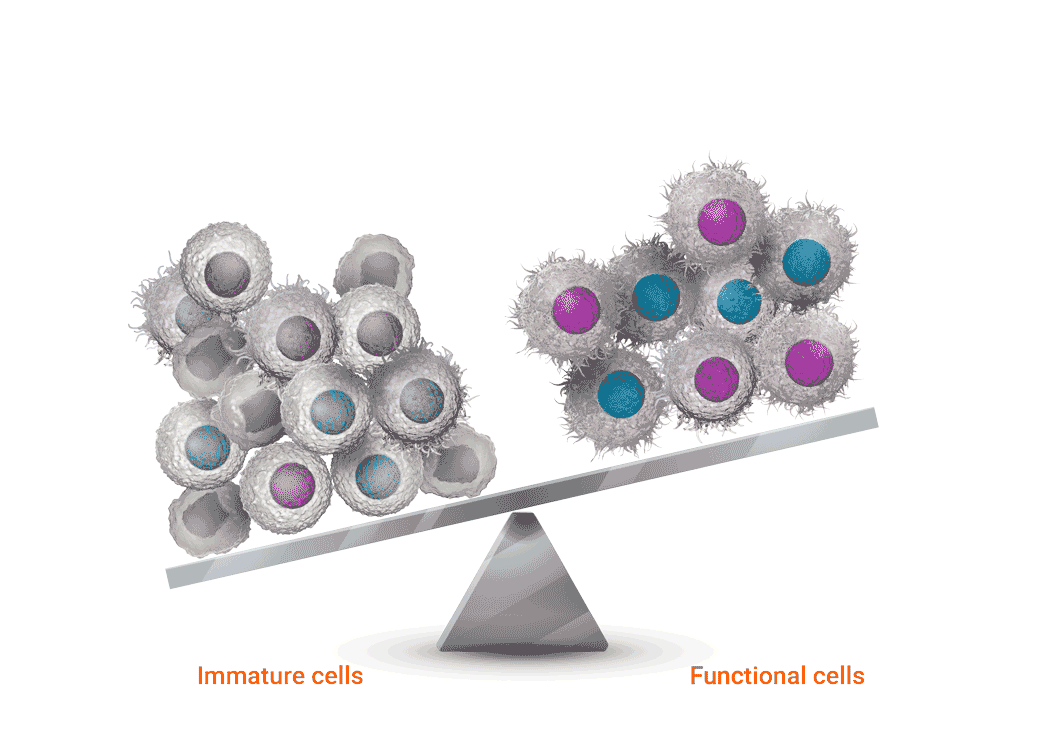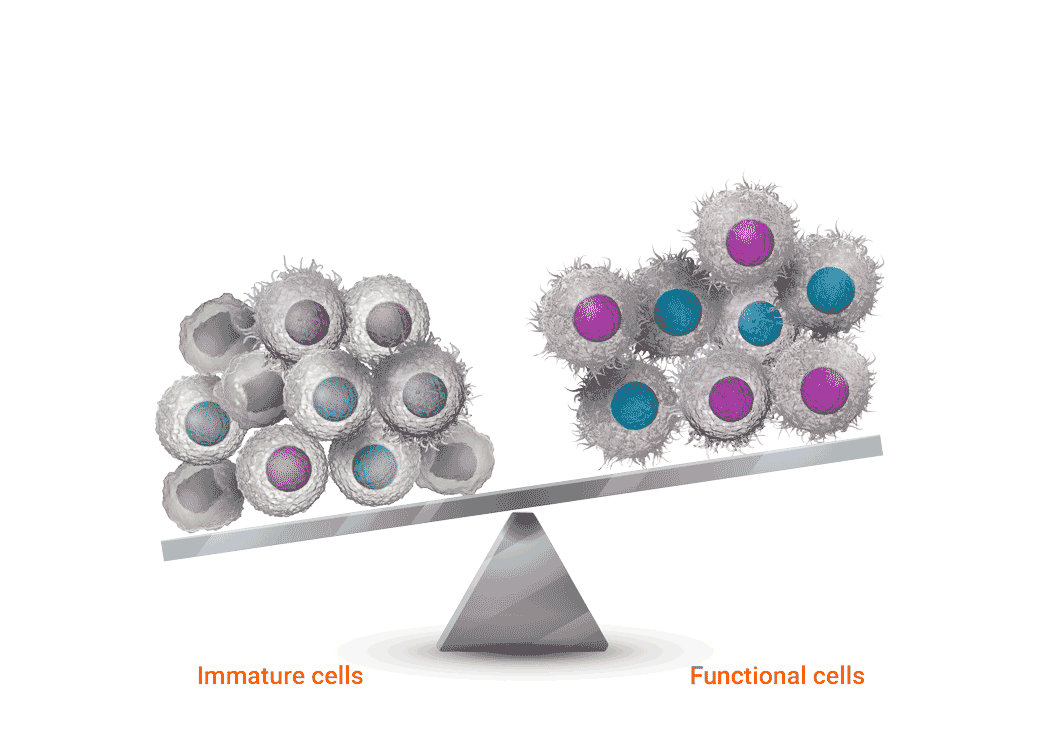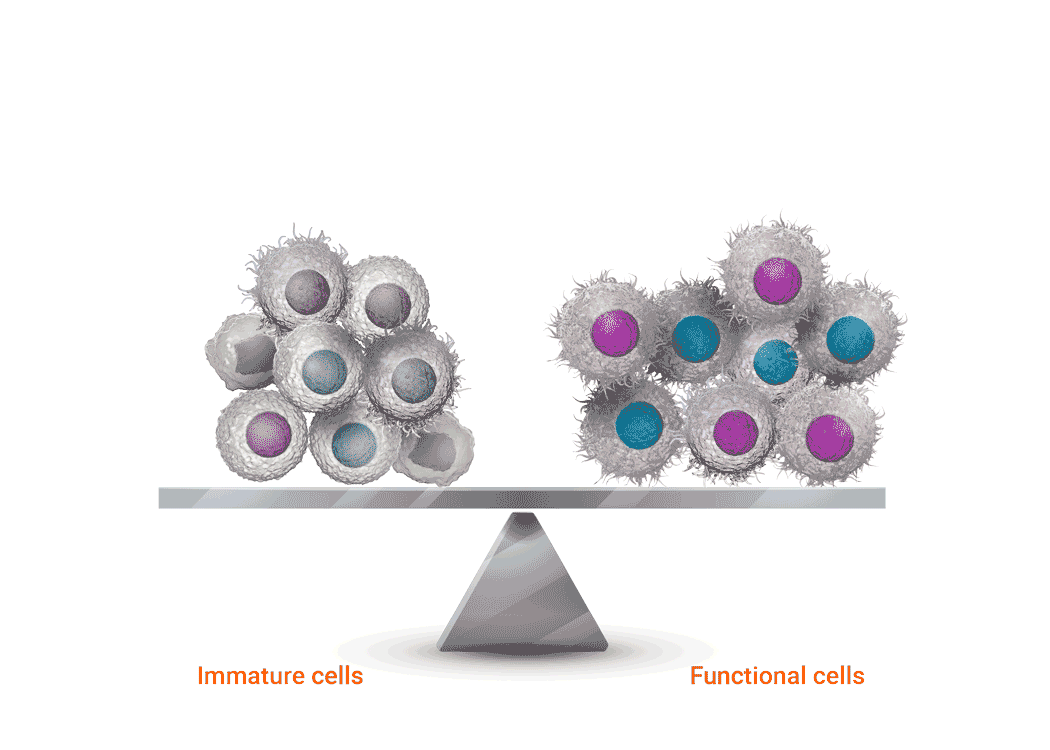

Joenja mechanism of action
Joenja and shows how Joenja addresses both immune deficiency AND
immune dysregulation to help normalize immune balance.




About APDS
pathway disrupts immune cell balance,
causing immune deficiency and
immune dysregulation.1,2,7,8
Clinical Data
efficacy and safety tolerability in a
Phase 3, randomized controlled
clinical trial.1
fimmu.2018.00543 3. Rao VK, Webster S, Dalm VASH, et al. Effective “activated PI3Kδ syndrome”—targeted therapy with the PI3Kδ inhibitor leniolisib. Blood. 2017;130(21):2307-2316. doi:10.1182/
blood-2017-08-801191 4. Data on file. Pharming Healthcare, Inc. 5. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605-635. doi:10.1016/j.cell.2017.07.029 6. Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3(4):317-330. doi:10.1038/nri1056 7. Rao VK, Webster S, Šedivá A, et al. A randomized, placebo-controlled phase 3 trial of the PI3Kδ inhibitor leniolisib for activated PI3Kδ syndrome. Blood. 2023;141(9):971-983. doi:10.1182/blood.2022018546 8. Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866-871. doi:10.1126/science.1243292