The Joenja clinical trials1-4
Part 1 Dose-finding
12 weeks
N=6
- Non-randomized, open-label dose-finding study in 6 patients
with APDS; dose range was 10 mg, 30 mg, and 70 mg BID for
4 weeks at each dose - Oral dose 70 mg BID selected for part 2

Part 2 Efficacy and
Safety Evaluation
Randomized period
12 weeks
N=31
- Randomized, triple-blinded (patient, caregiver, investigator), placebo-controlled, fixed-dose study of 70 mg BID
-
Co-primary efficacy end points (improvement in lymphoproliferation and normalization of immunophenotype)
- Change from baseline in the log10-transformed SPD of index lesions
- Change from baseline in percentage of naïve B cells out of total
B cells
- Secondary and exploratory end points
- Safety
Open-label extension (OLE) study
N=37
-
An open-label, non-randomized extension study to evaluate the long-term safety, tolerability, efficacy, and pharmacokinetics of Joenja in patients with APDS
- Thirty-five patients from parts 1 and 2
- Two patients previously treated with another investigational PI3Kδ inhibitor
- Primary outcome measure: long-term safety and tolerability
Joenja was studied in patients with confirmed PI3Kδ variants1,5
in patients with APDS

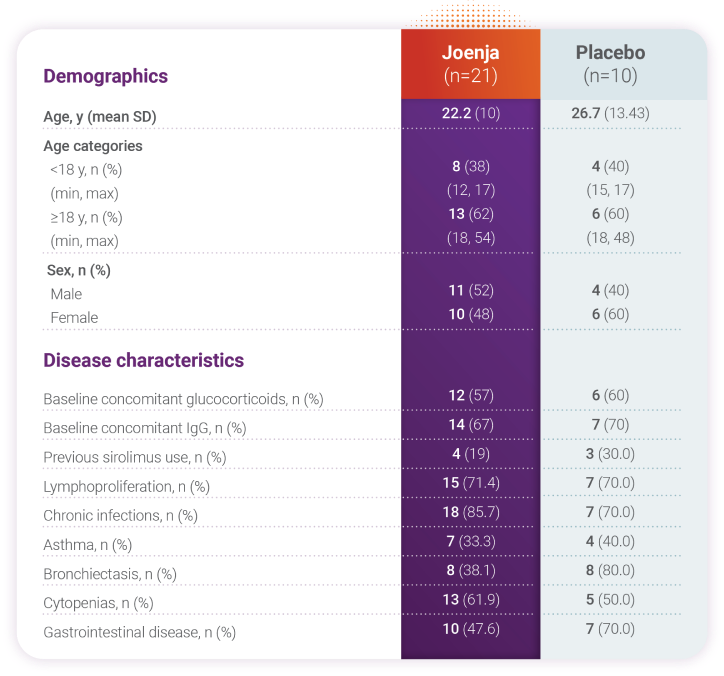
IgG, immunoglobulin G; IRT, immunoglobulin replacement therapy; SD, standard deviation.
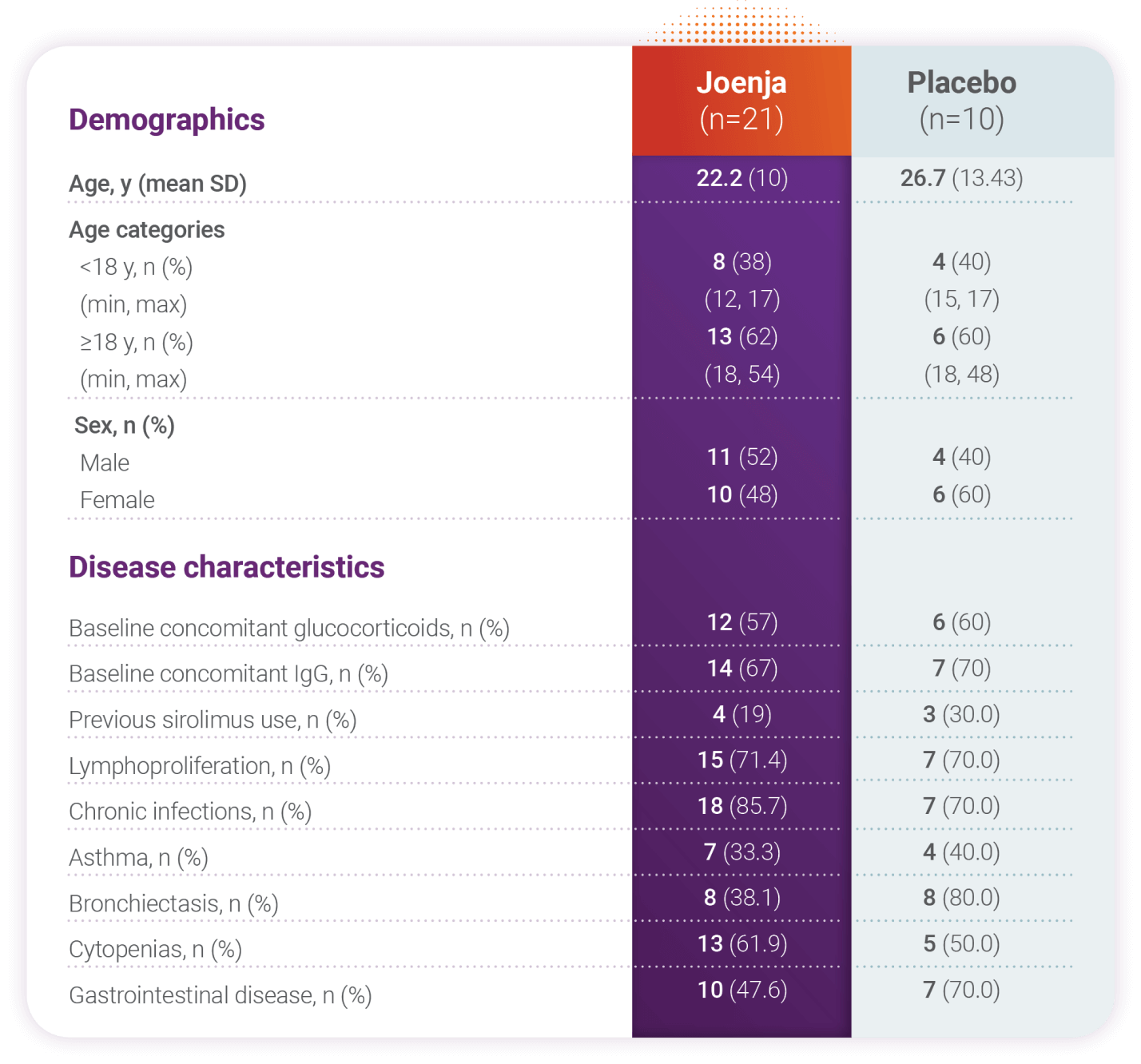
Patients had nodal and/or extranodal lymphoproliferation, as measured by index nodal lesion selected by the Cheson methodology on CT or MRI and clinical findings and manifestations compatible with APDS (eg, history of repeated oto-sino-pulmonary infections, organ dysfunction). Immunosuppressive medications or PI3Kδ inhibitors (selective or non-selective) were prohibited within 6 weeks of baseline (day -1 and the visit prior to first study drug administration) and throughout the study. In addition, patients who had previous or concurrent B cell depleters (eg, rituximab) within 6 months of baseline were excluded from the study unless absolute B lymphocytes in the blood were normal. B cell depleters were prohibited throughout the study.About APDS
Hyperactivity along the Pl3Kδ signaling
pathway disrupts immune cell balance,
causing immune deficiency and
immune dysregulation.1,5-7
Clinical Data
efficacy, safety, and tolerability in
Phase 3, randomized controlled
clinical trial.1
5. Rao VK, Webster S, Šedivá A, et al. A randomized, placebo-controlled phase 3 trial of the PI3Kδ inhibitor leniolisib for activated PI3Kδ syndrome. Blood. 2023;141(9):971-983. doi:10.1182/blood.2022018546 6. Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866-871. doi:10.1126/science.1243292 7. Maccari ME, Abolhassani H, Aghamohammadi A, et al. Disease evolution and response to rapamycin in activated phosphoinositide 3-kinase δ syndrome: The European Society for Immunodeficiencies-Activated Phosphoinositide 3-Kinase δ Syndrome Registry. Front Immunol. 2018;9:543. doi:10.3389/fimmu.2018.00543